
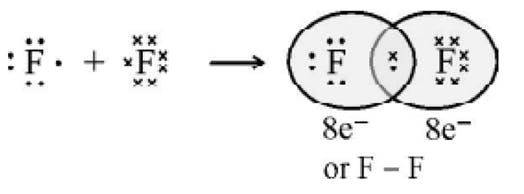
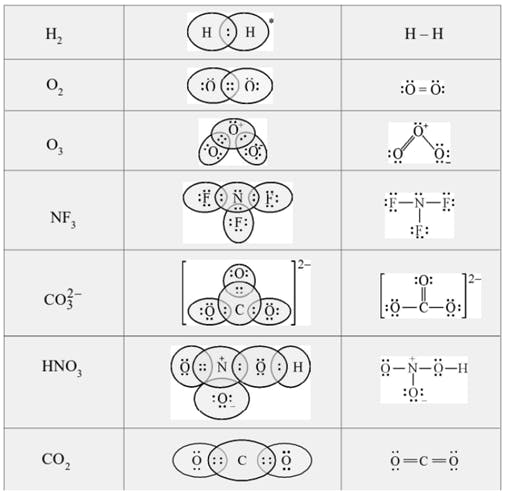
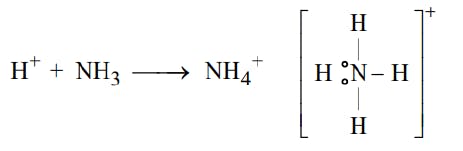
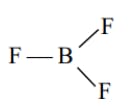
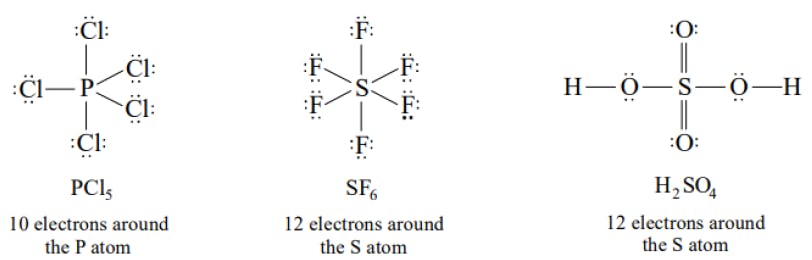
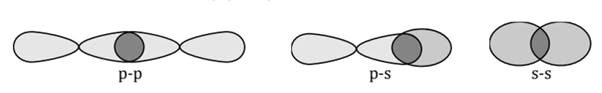
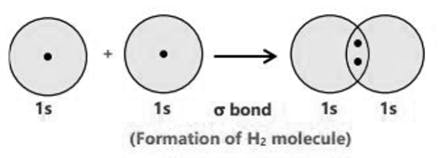
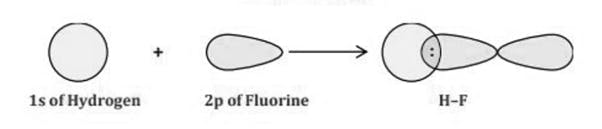
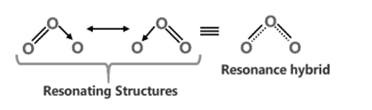
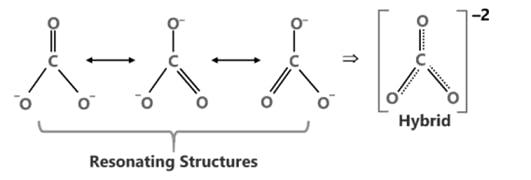
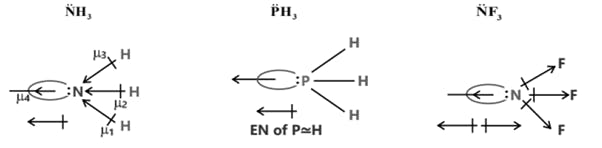
The Lewis Representation of some molecules is as follows
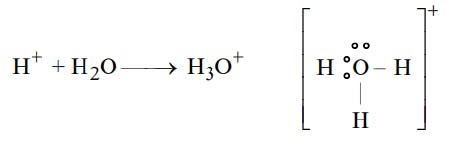
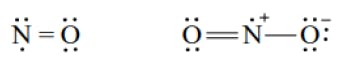
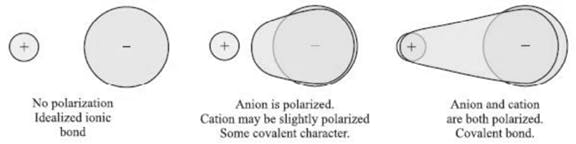
Covalent Character in Ionic Bonds
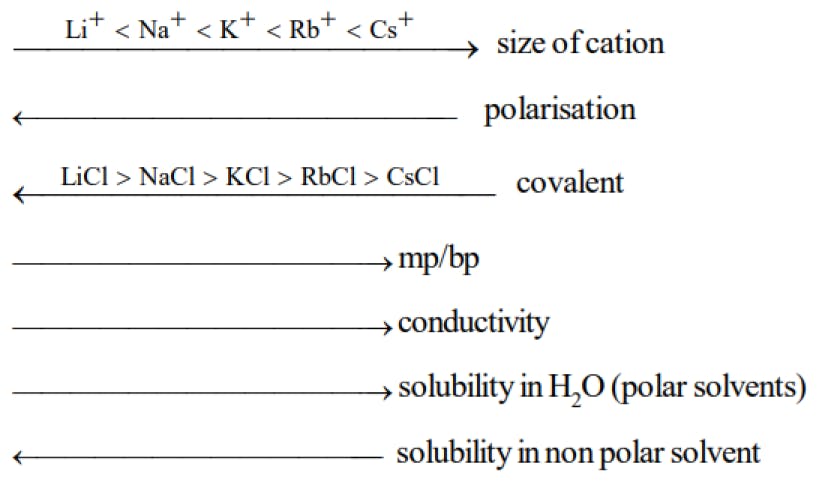
When a cation is placed near an anion, it distorts the spherical shape of the anionic electron cloud due to its attractive force. Such a distorted anion is said to be polarized.
Since the electrons now have a better tendency to stay between the two nuclei rather than entirely on the anion, the ionic compound is said to have acquired a certain covalent character. The tendency of the cation to polarize the anion is called its polarizing power.
The tendency of the anion to undergo polarization is called its polarizability.
Greater the polarizing power of the cation or polarizability of the anion, greater is the covalent character of the molecule.
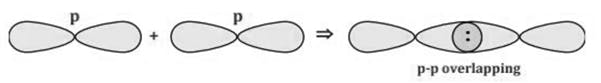
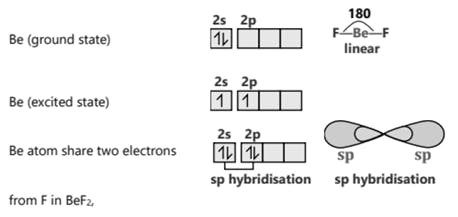
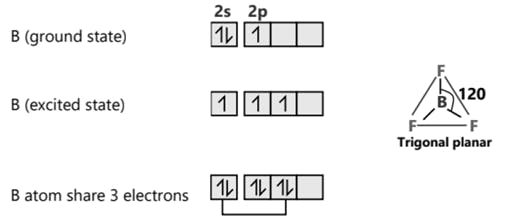

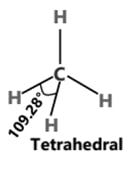

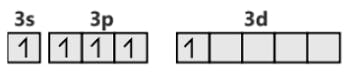
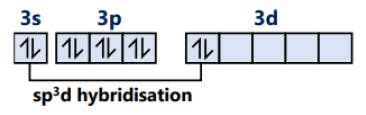
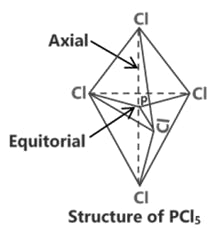
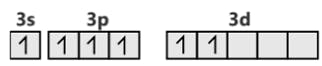
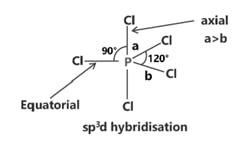
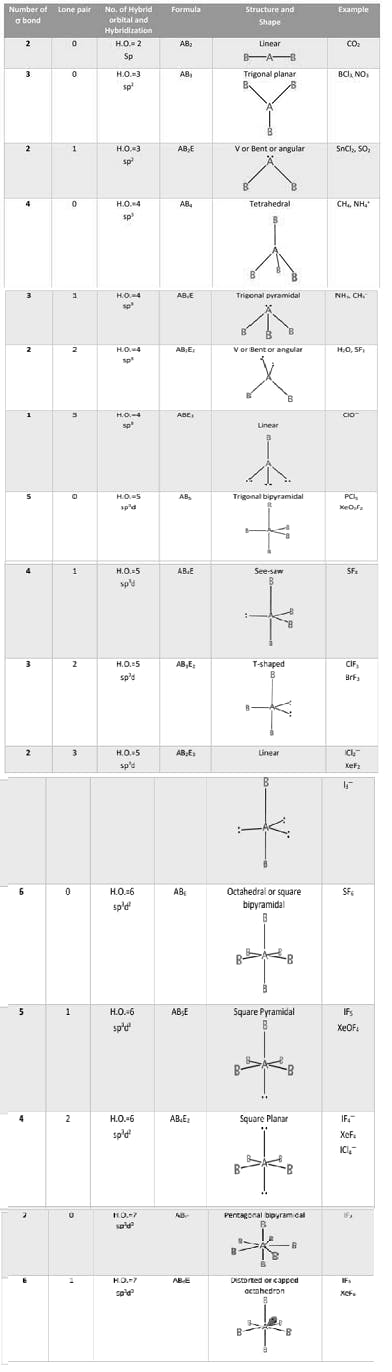
SF6 S (ground state)
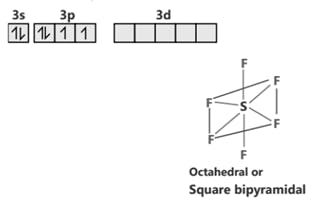
S (excited state)
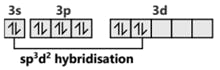
S (after hybridisation)


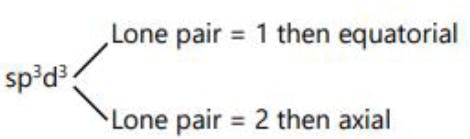
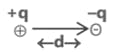
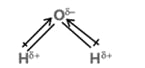
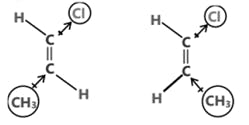
(c) Dipole moment is a vector quantity i.e. it has both magnitude as well as direction.
(d) Direction of dipole moment is represented by an arrow pointing from electro +ve to electro -ve element and from central atom to lone pair of electrons.
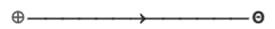
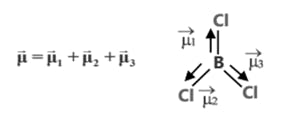
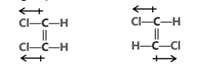
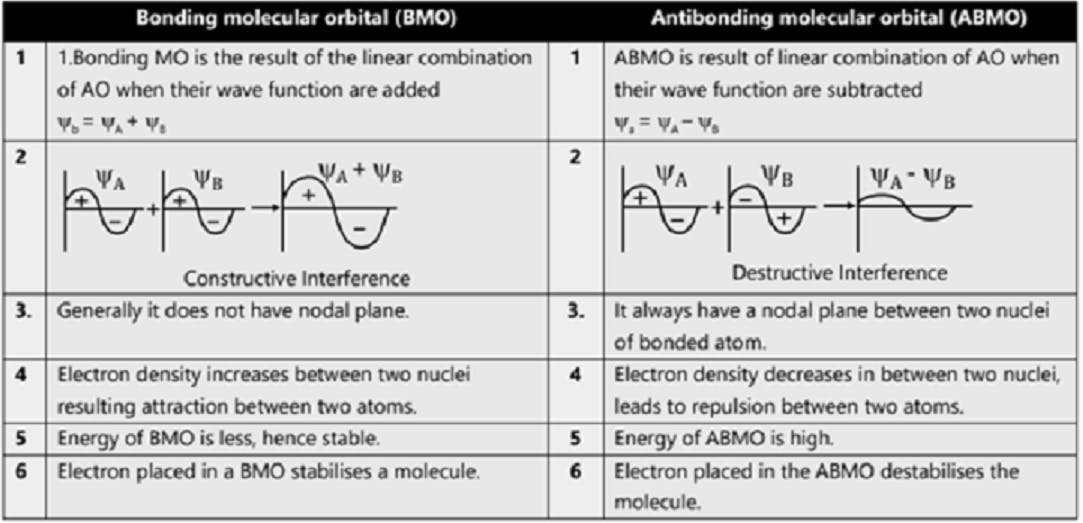
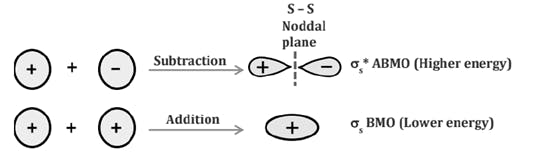
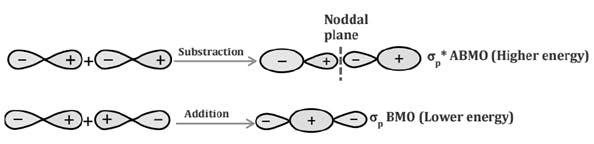
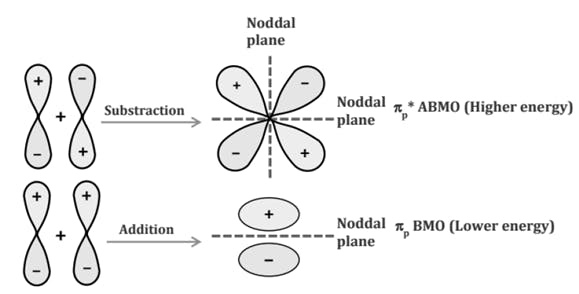
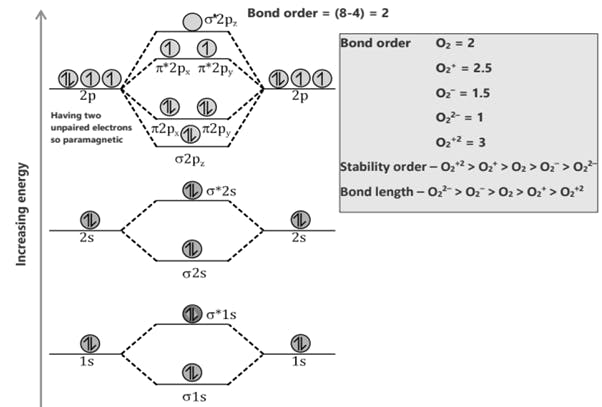
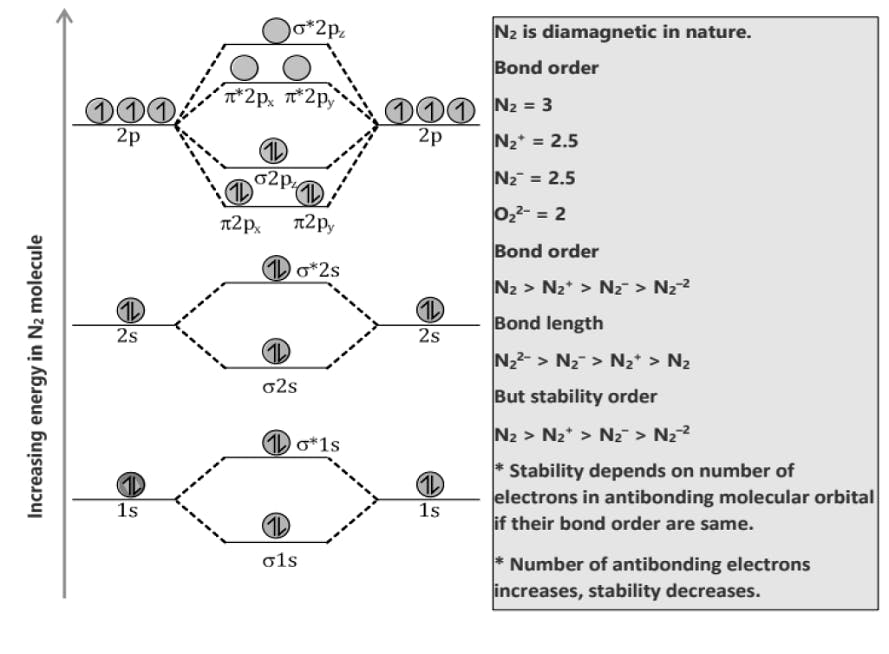
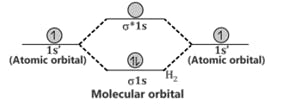
Comparison of Bonding molecular orbital & Antibonding molecular orbital:
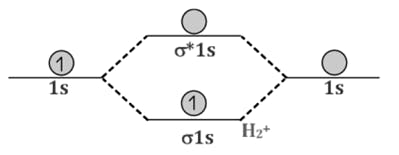
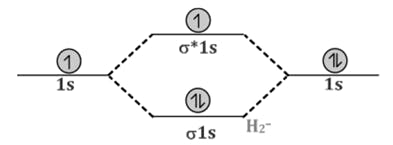
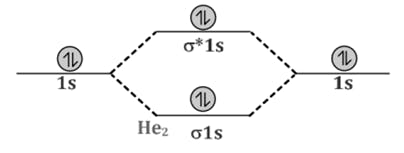
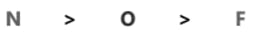
Decreasing order of electronegativity is -

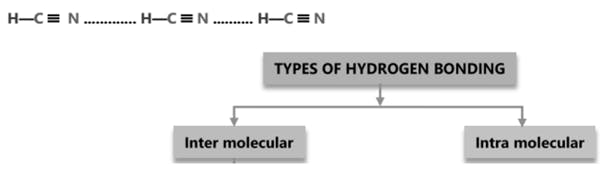

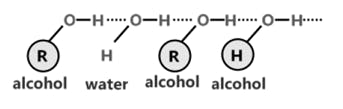
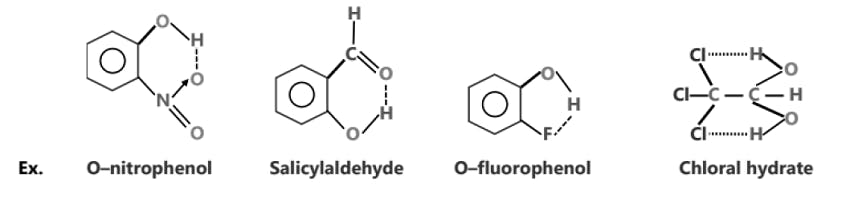
Intramolecular H-bond
It takes place within the molecule.
(a) H -bonded with electronegative element of a functional group, form H -bond with another electronegative element present on nearest position on the same molecule.
(b) This type of H -bond is mostly occurred in organic compounds.
(c) It results in ring formation (Chelation).
(d) Less association in comparison to inter molecular H -bond, so it will have less strength
Van der waal's forces
(a) These are weak, non-directional, non-valence force of attraction among neutral species.
(b) These are electrical in nature, due to induced polarity caused by temporary displacement of electrons towards one end of the inert atoms, becoming a temporary dipole.
(c) This temporary dipole in one molecule can induce opposite dipoles in surrounding molecule due to displacement of electrons, one end becomes
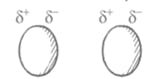
Negative and other Positive. These partially charged ends, induce surrounding molecules accordingly.
(d) Strength of Van der Waal force depends on ease of distortion of electron cloud.
Chemistry Faculty
B.Tech from IIIT
IIIT Unnao. 8+ Years of experience as Chemistry Faculty